Editorial: Geography – an increasingly important variable in prostate cancer clinical trials
Enzalutamide is approved in the post-docetaxel phase for men with metastatic castrate-resistant prostate cancer (mCRPC) in North America (NA) and the European Union (EU). Merseburger et al. [1] now take a first look at comparing the efficacy and safety of enzalutamide in EU and NA patients treated in the AFFIRM trial. AFFIRM was a phase III double-blind, placebo-controlled multinational trial evaluating whether enzalutamide prolongs overall survival (OS) in men with mCRPC after docetaxel chemotherapy. This post hocexploratory analysis found a consistent OS benefit in both NA- and EU-treated patients. The safety profile was comparable in both regions.
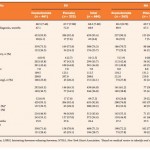
Interestingly, the median OS appeared longer in the EU as compared with the NA cohort (‘not yet reached’ vs 17.4 months). With the placebo group in the EU the median survival was 16.2 vs 12.3 months in the NA cohort (3.9 months different). Direct statistical comparisons between regions were not performed but a median survival difference of 3.9 months is not inconsequential. Notably, drugs have been approved with lesser difference in OS.
The populations in AFFIRM have some baseline differences that may be of importance; the median time from diagnosis to randomisation was 68.9 months in the EU cohort vs 78.0 months in the NA cohort. Thus, it was possible that the NA cohort was ‘further along’ in the natural history of the disease despite apparently being well-balanced according to most baseline variables. The NA cohort was slightly more likely to have higher Gleason scores (50.6%) vs the EU cohort (44.0%), suggesting perhaps a slight difference in disease aggressiveness. It is also interesting that cardiac disease was present in 23.3% of the NA cohort vs only 7.2% of the EU cohort. Is it possible that cardiovascular diseases also contributed to a relative increase in mortality in the NA cohort? With regard to the post-protocol therapies, the use of abiraterone was more common in the EU cohort (30.5% of patients in the placebo group subsequently received abiraterone in the EU cohort vs 19.7% in the NA cohort). However, utilisation of cabazitaxel was more common in NA than in the EU (20.5% vs 9.9%). Some combination of pre-treatment and post-protocol variables in AFFIRM is probably the explanation for geographic variations in OS. Protocol treatments were well defined but these variables were not controlled.
These results in AFFIRM should also be considered in the context of geographic differences in medical care that may alter results and/or interpretation of phase III trials. Some of these differences have been previously documented in other phase III mCRPC trials. For instance, the recent phase III trials with orteronel reported no OS differences in the overall study [2]. However, in certain geographic regions (with less access to the newer life-prolonging drugs), the orteronel trials were clearly positive for the OS endpoint. In the TROPIC trial, cabazitaxel was associated with higher febrile neutropenia rates in some EU countries as compared with the USA. This was primarily due to geographic differences in prophylactic administration of granulocyte colony-stimulating factor (G-CSF) [3]. For the ALSYMPCA trial, striking geographic differences were noted in the timing (pre- or post-docetaxel) of radium-223 utilisation [4].
Understanding prognosis and treatment outcomes is increasingly critical in the design and interpretation of phase III clinical trials and particularly amongst trials that are designed to support regulatory approvals. Region or country where treatment takes place is an important variable to consider, especially if approved pre- and post-protocol treatments are not the same. This issue is even more important if the course of the disease is long (as in prostate cancer) and there are different treatments available from country to country.
Oliver Sartor, Sumanta Kumar Pal*, Terhi Hermanson† and Charles L. Bennett‡,
Tulane Medical School, New Orleans, LA, *City of Hope Comprehensive Cancer Center, Duarte, CA, USA; † Helsinki University Central University, Helsinki, Finland; and ‡ College of Pharmacy, Medical University of South Carolina Cancer Center, Charleston, SC, USA
References
1 Merseburger AS, Scher HI, Bellmunt J et al. Enzalutamide in European and North American men participating in the AFFIRM trial. BJU Int 2015; 115: 41–9
2 Fizazi K, Jones R, Oudard S et al. Regional differences observed in the phase 3 trial (ELM-PC 5) with orteronel (TAK-700) plus prednisone in patients with metastatic castration-resistant prostate cancer (mCRPC) that has progressed during or following docetaxel. ASCO Meeting Abstracts. J Clin Oncol 2014; 32 (Suppl.): 5s (abstr 5042). Available at: https://meetinglibrary.asco.org/content/126314-144. Accessed November 2014
3 Ozguroglu M, Oudard S, Sartor AO et al. Effect of G-CSF prophylaxis on the occurrence of neutropenia in men receiving cabazitaxel plus prednisone for the treatment of metastatic castration-resistant prostate cancer (mCRPC) in the TROPIC study. ASCO Meeting Abstracts. J Clin Oncol 2011; 29 (Suppl.): abstr 144. Available at: https://meetinglibrary .asco.org/content/72386-104. Accessed November 2014
4 Parker C, Nilsson S, Heinrich D et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369: 213–23